Disorder Definitions
The following definitions are provided as a reference for physicians.
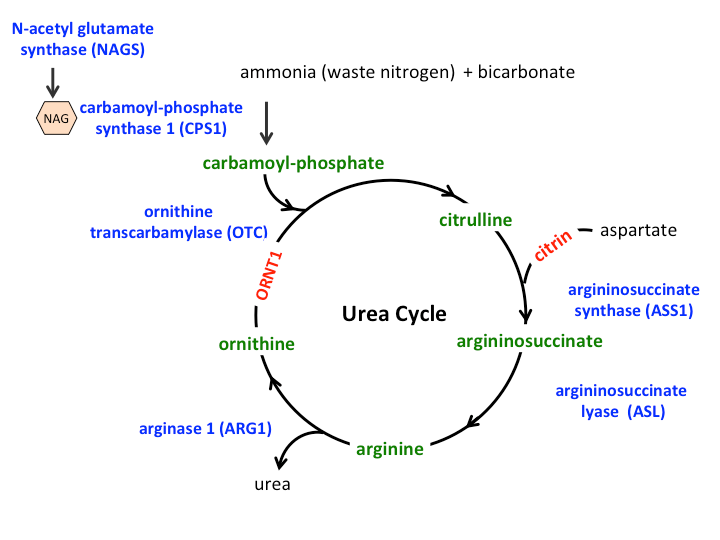
The figure of the urea cycle illustrated here shows the role of the enzymes (depicted in blue) and transporters (depicted in red) in conversion of ammonia nitrogen into urea. The nitrogen from ammonia and aspartate is “handed over” to a number of intermediate compounds (depicted in green) before finally being converted into urea. A deficiency of any of the enzymes or transporters of the urea cycle leads to an inability or reduced ability to dispose of nitrogen and accumulation of ammonia.
N-acetylglutamate Synthase (NAGS) Deficiency
The enzyme NAGS makes a molecule called N-acetylglutamate, which is essential for the functioning of the first urea cycle enzyme, CPS1. Patients with complete NAGS deficiency develop high ammonia levels in the blood (hyperammonemia) soon after birth. Patients who are successfully rescued from high ammonia are at risk for further episodes of hyperammonemia. Patients with partial NAGS deficiency (milder type of NAGS) can have symptoms that appear at any time of life with triggering events such as an infection or other stress. NAGS deficiency is typically diagnosed by genetic testing. NAGS deficiency is the only UCD in which the hyperammonemia can be completely reversed by a medication called Carglumic acid.
Carbamoyl-phosphate Synthase 1 (CPS1) deficiency
CPS1 is an enzyme that is important for the first step of the urea cycle that converts ammonia into a compound called carbamoyl-phosphate. Patients with complete CPS1 deficiency rapidly develop hyperammonemia soon after birth. Patients who are successfully rescued from hyperammonemia are at risk for repeated episodes of hyperammonemia. Patients with partial CPS1 deficiency (milder type) can have symptoms appear at any time of life with a triggering event such as an infection or other stress.
CPSI is the first enzyme in the urea cycle and is found primarily in the liver and the intestine. Currently, diagnosis is based on DNA testing for mutations in CPS1 gene or enzymatic assay on liver tissue.
Treatment consists of restriction of protein intake, use of essential amino acid supplements, supplementation of L-citrulline, and diversion of nitrogen by alternate pathway therapy with sodium benzoate and/or sodium phenylbutyrate. Liver transplantation is indicated in hard to control severe cases.
Ornithine Transcarbamylase (OTC) Deficiency
The enzyme OTC combines carbamyl-phosphate that is produced by CPS1 with an amino acid called ornithine to make citrulline. Patients with complete OTC deficiency (the most severe type of this disorder) rapidly develop high levels of ammonia in the blood, soon after birth and develop symptoms any time before one week of age. Infants who are successfully rescued from this first crisis are at risk for repeated episodes of hyperammonemia. The OTC gene is located on the X-chromosome. Males have only one X-chromosome while females have two. Hence, the majority of patients with severe presentations of OTC deficiency are males. Females with one “abnormal OTC gene” and one “normal OTC gene” may not show any clinical evidence of the disorder; however 15% can show some symptoms or signs of the disease. Patients with partial OTC deficiency (milder type of OTC) can present at any time of life with a triggering event such as an infection or other stress. The rise in ammonia levels is generally lower in females with this disorder as compared to the males.
The diagnosis of OTC deficiency relies mostly on DNA analysis for mutations, however, in approximately 10% of patients a deleterious mutation cannot be found, requiring enzyme analysis on a liver biopsy to establish the diagnosis.
Treatment consists of restriction of protein intake, use of essential amino acid supplements, supplementation of L-citrulline, and diversion of nitrogen by alternate pathway therapy with sodium benzoate and/or sodium phenylbutyrate. Liver transplantation is indicated in hard to control severe cases.
Argininosuccinate Synthase (ASS) Deficiency (also known as Citrullinemia type I)
The enzyme ASS1 (or ASS) uses the citrulline produced by OTC and combines it with the amino acid aspartate to make a compound called argininosuccinate. Patients with complete deficiency of ASS (most severe type of this disorder) present with high levels of ammonia soon after birth. The blood level of citrulline in these patients is typically many times higher than the normal.
Although plasma amino acid analysis in the context of hyperammonemia is sufficient for reliable diagnosis, further diagnostic confirmation can be obtained by DNA analysis of mutations and/or enzymatic assay on cultured skin fibroblasts. Prenatal testing is performed by DNA testing and/or enzymatic analysis on amniocytes or CVS sample.
Treatment consists of restriction of protein intake, use of essential amino acid supplements, supplementation of L-arginine (not L-citrulline which is contraindicated) and diversion of nitrogen by alternate pathway therapy with sodium benzoate and/or sodium phenylbutyrate. Liver transplantation is indicated in hard to control severe cases.
Citrin Deficiency (also called Citrullinemia type II)
Citrin is a protein that is needed to transport the amino acid aspartate into the urea cycle. This type of protein is called a transporter. Adults with citrin deficiency (also called citrullinemia type II) can present with hyperammonemia, and cyclical behavior abnormalities including aggression, irritability, and hyperactivity, as well seizures, and coma. Infants and children with citrin deficiency present differently and can have abnormalities of liver and failure to thrive. These patients can also have the dietary peculiarity of avoiding sugars rather than protein (which most urea cycle patients avoid). The majority of patients reported have been Japanese or Asian who share common mutations in the Citrin gene.
The diagnosis is confirmed by DNA analysis of mutation in the citrin gene almost all of which are of the null type (no protein is being made).
These patients respond better to high protein low carbohydrate diet unlike the other urea cycle disorders which are treated with low protein high carbohydrate diets.
Argininosuccinate Lyase (ASL) Deficiency (also known as Argininosuccinic Aciduria)
Argininosuccinate lyase is an enzyme that is needed to breakdown a compound in the urea cycle called argininosuccinate. Patients with complete deficiency of ASL (most severe type of this disorder) present soon after birth with high levels of ammonia. Those with milder forms of deficiency may present later in childhood with hyperammonemia during stress or infections. Patients can also develop trichorrhexis nodosa, a fragile hair abnormality. Patients with this condition can also develop abnormalities of the liver. Some affected patients who have never had severe hyperammonemia can also demonstrate some developmental disabilities. The levels of argininosuccinic acid in the blood and urine are high in patients (hence the name argininosuccinic aciduria).
The specific diagnosis is based on the finding of large amounts of argininosuccinic acid and its anhydrates in the blood and urinee and/or DNA testing for mutations or direct enzymatic analysis on fibroblasts. Prenatal diagnosis is available by DNA analysis and/or enzymatic analysis of amniocytes or CVS sample.
Treatment consists of restriction of protein intake, use of essential amino acid supplements, administration of L-arginine with or without diversion of nitrogen by alternate pathway therapy with sodium benzoate and/or sodium phenylbutyrate. Liver transplantation is indicated in hard to control severe cases and those with severe liver disease.
Arginase (ARG) Deficiency (also known as hyperargininemia)
Arginase is the last enzyme of the urea cycle that breaks down the amino acid arginine produced by the urea cycle, into two molecules, urea and ornithine. The urea is disposed of by the kidneys; this is the way nitrogen from ammonia is excreted by the body. Arginase deficiency is typically not characterized by severe increase in ammonia. Patients often present with progressive problems of muscle control. They can also develop seizures and have developmental disabilities. Growth is usually slow and without therapy they usually do not reach normal adult height. Other symptoms that may present early in life include episodes of irritability, poor appetite, and vomiting. Severe episodes of hyperammonemia can occur but are infrequent.
The specific diagnosis relies on finding elevated levels of arginine in the blood and by analysis of enzymatic activity in red blood cells and/or DNA analysis for mutations.
Treatment is similar to other urea cycle disorders (except arginine is of course contraindicated), with restriction of protein intake, use of essential amino acid supplements and diversion of nitrogen by alternate pathway therapy with sodium benzoate and/or sodium phenylbutyrate.
Ornithine Translocase Deficiency (also known as hyperornithinemia-hyperammonemia-homocitrullinuria or HHH Syndrome)
Ornithine translocase is a transport protein that moves ornithine and citrulline molecules within the urea cycle. When this transport does not work properly, it causes the urea cycle to slow down and ammonia builds up in the blood. A number of other molecules also build up including ornithine in the blood and homocitrulline in the urine. Most patients have episodic hyperammonemia, accompanied by vomiting, sleepiness and (in extreme cases) coma. Growth is abnormal and learning can be affected. Common symptoms include seizures and muscle control problems. Patients that have partial activity of the transporter (mild type of disorder) have symptoms beginning in adulthood. They typically self-select low protein diets without being aware they have this disorder.
Diagnosis can be made based on elevated ornithine levels in the plasma and elevated homocitrulline levels in the urine.
Treatment consists of restriction of protein intake, supplementation of citrulline and diversion of nitrogen by alternate pathway therapy with sodium benzoate and/or sodium phenylbutyrate.

